Getting Helium: The Journey of a Unique Element

Hey there, science enthusiasts! Have you ever looked up at a balloon sailing high in the sky or watched a bright, colorful party decoration slowly losing its lift? If that balloon floats effortlessly, it’s probably filled with helium, one of the coolest and most fascinating elements on the periodic table! But have you ever wondered how we actually get helium? Let’s dive into the science behind this amazing gas!
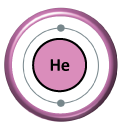
First, let’s explore what helium actually is. Helium is a colorless, odorless, and tasteless gas, and it’s the second lightest element after hydrogen. It is represented by the symbol “He” on the periodic table and has an atomic number of 2. One of the most remarkable things about helium is that it is a noble gas, meaning it doesn’t react easily with other elements. This property makes helium perfect for various applications, including balloons, deep-sea diving, and even scientific research.
Helium has a fascinating origin story that dates back to the universe’s birth. Most of the helium on Earth didn’t actually come from our planet! Helium was formed during the Big Bang around 13.8 billion years ago. The intense heat and pressure produced during those early moments led to the fusion of hydrogen atoms, resulting in isotopes that eventually became helium.
In addition to its cosmic origins, helium can also be found on Earth, although it’s rare. Most helium comes from natural gas deposits located underground, typically in association with highly radioactive minerals. Scientists estimate that Earth contains about 0.00052% helium in its atmosphere, but this amount is incredibly small compared to more abundant gases like nitrogen and oxygen.
So, how do we get helium from natural gas? The extraction process is pretty interesting! When natural gas is drilled from deep within the Earth, it contains a variety of gases, including methane (the main component of natural gas), nitrogen, and, of course, helium. To separate helium from other gases, companies use a method called cryogenic distillation.
In this method, the natural gas is cooled to very low temperatures, which causes the various gases to liquefy at different rates. Helium, being so light, remains in a gaseous state while other gases turn into liquid. Once the different gases are separated, helium can be collected and further purified for use.
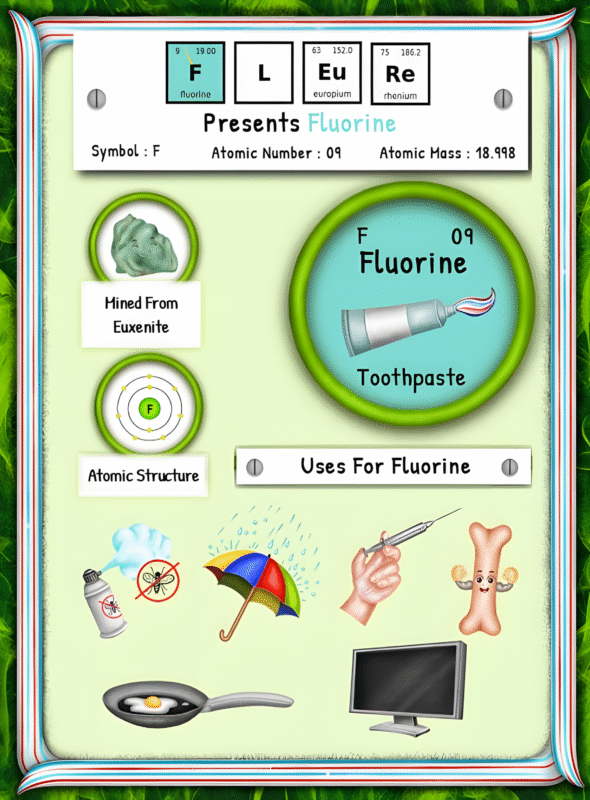
Once extracted, helium is incredibly useful! It’s commonly used to fill party balloons, making them float. But it doesn’t stop there! Helium is also essential in the medical field; it cools MRI machines and helps create a super-cooled environment for scientific experiments. Additionally, it’s used in the aerospace industry for rocket testing and in the production of fiber optics.
In conclusion, helium is more than just a party favorite. It’s an element with a rich background, originating from the cosmos and being extracted from deep Earth resources. Its unique properties make it invaluable in various industries. So, the next time you see a balloon floating or hear about the use of helium in a medical setting, remember the incredible journey of this element from the stars to your hands! Science is truly amazing, and knowing how we obtain helium is just one piece of the puzzle. Keep exploring, and who knows what you’ll discover next!
This article is brought to you by Sybrina Durant, the author of the middle grade picture book, Magical Elements of the Periodic Table Presented Alphabetically By The Elemental Wizards. Learn More. In that book Helium is presented by the Wizard, Hetha.
Inter-Active Elemental Fantasy-Themed Periodic Table from Magical Elements of the Periodic Table Presented Alphabetically by The Elemental Dragon Clan
Click here to use This Inter-Active Viewer To Learn More About The Elements Each Elemental Represents On This Periodic Table. Want this in a 24″ x 36″ Poster? Click here.
Sybrina Publishing Offers Fun Activities Based On The Book
Magical Elements of the Periodic Table Magical Elementals
Browse Magical Elemental Activities at MagicalPTElements or Sybrina-Publishing on TPT or Classful