The Early Days of Deuterium: Pioneering Uses and its Evolving Role
When it comes to hydrogen isotopes, most of us are familiar with protium, the lightest and most common form. However, there is another isotope, deuterium, that has played a significant role in scientific research and practical applications since its discovery in 1931. Its initial applications were groundbreaking, yet over time, deuterium was gradually replaced by more efficient alternatives. Let us delve into its early uses and explore the reasons behind its decline from those specific purposes.
Deuterium Emerges:
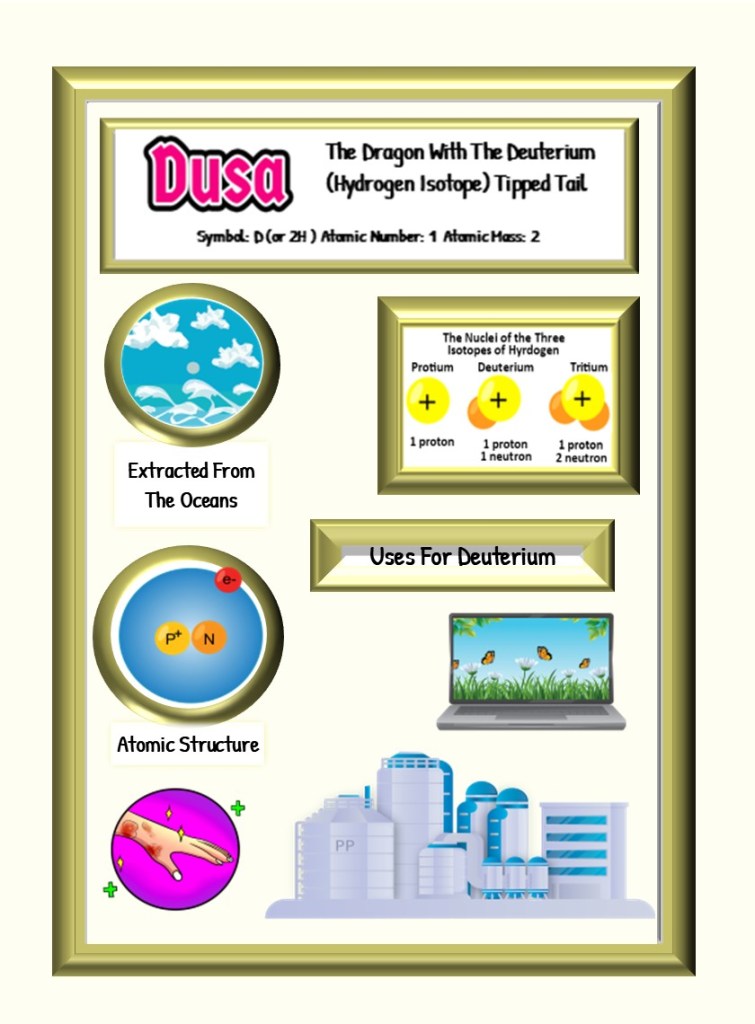
The story of deuterium begins with its discovery by the American chemist and physicist Harold Urey in 1931. Urey successfully separated the isotopes of hydrogen while investigating the differences in their masses. Deuterium, also known as “heavy hydrogen,” contains an extra neutron, making it twice as heavy as common protium.
- Nuclear Research:
One of the earliest uses of deuterium was in nuclear research. Scientists utilized deuterium as a neutron moderator, slowing down neutrons to create controlled nuclear reactions. It was particularly useful in the development of the first nuclear weapons, enabling scientific understanding and design refinement. - Nuclear Fusion:
Deuterium proved instrumental in early experiments on nuclear fusion, the process that powers the sun. Researchers attempted to harness this immense source of energy for peaceful use on Earth. Deuterium, in combination with tritium (a heavier isotope of hydrogen), served as a fusion fuel source in early fusion reactors. The fusion reaction between deuterium and tritium releases vast amounts of energy, making the use of deuterium essential in early experimental reactors.
Despite its early accomplishments, deuterium faced several limitations, leading to its gradual replacement for these specific purposes.
- Availability and Cost:
Deuterium is relatively rare, representing only a small fraction (about 0.015%) of naturally occurring hydrogen. Extracting and isolating deuterium is an expensive and complex process, making it less economical compared to other isotopes or renewable energy sources like solar power and wind energy. As a result, the high cost hindered its continued use in expansive applications. - Safety and Handling Concerns:
Deuterium and deuterium-tritium fusion reactions produce energetic neutrons, posing safety concerns for researchers and operators. Managing the intense radiation emitted during nuclear fusion experiments requires complex systems and shielding. In contrast, other fusion fuels like hydrogen-boron (an isotope of boron), which produces fewer neutrons during the reaction, gained attention for their inherently safer characteristics. - Technological Advancements:
Over time, the field of nuclear research witnessed significant advancements. Researchers have developed and focused on alternative fuel sources, such as hydrogen-boron fusion, compact fusion reactors, and advancements in nuclear fission technology. These alternatives have shown great potential in terms of energy efficiency, feasibility, and safety, rendering the earlier use of deuterium less attractive.
Deuterium, the heavy hydrogen isotope, played a pivotal role in the early stages of nuclear research and the exploration of fusion as an energy source. However, due to limitations such as increased cost, safety concerns, and advancements in alternative technologies, deuterium lost its prominence in those specific areas. Nonetheless, deuterium continues to find applications in fields like nuclear magnetic resonance spectroscopy and heavy water production, maintaining its significance in various scientific and industrial domains.
This article is brought to you by Sybrina Durant, the author of the middle grade picture book, Magical Elements of the Periodic Table Presented Alphabetically By The Elemental Dragons. Learn More. In that book Deuterium is presented by the dragon, Dusa.
Inter-Active Elemental Fantasy-Themed Periodic Table from Magical Elements of the Periodic Table Presented Alphabetically by The Elemental Dragon Clan
Click here to use This Inter-Active Viewer To Learn More About The Elements Each Elemental Represents On This Periodic Table. Want this in a 24″ x 36″ Poster? Click here.
Sybrina Publishing Offers Fun Activities Based On The Book
Magical Elements of the Periodic Table Magical Elementals
Browse Magical Elemental Activities at MagicalPTElements or Sybrina-Publishing on TPT or Classful