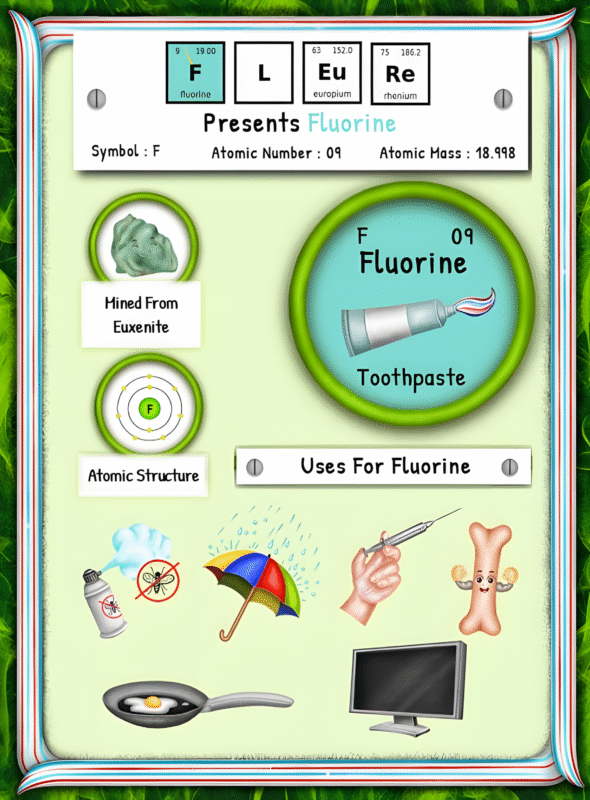
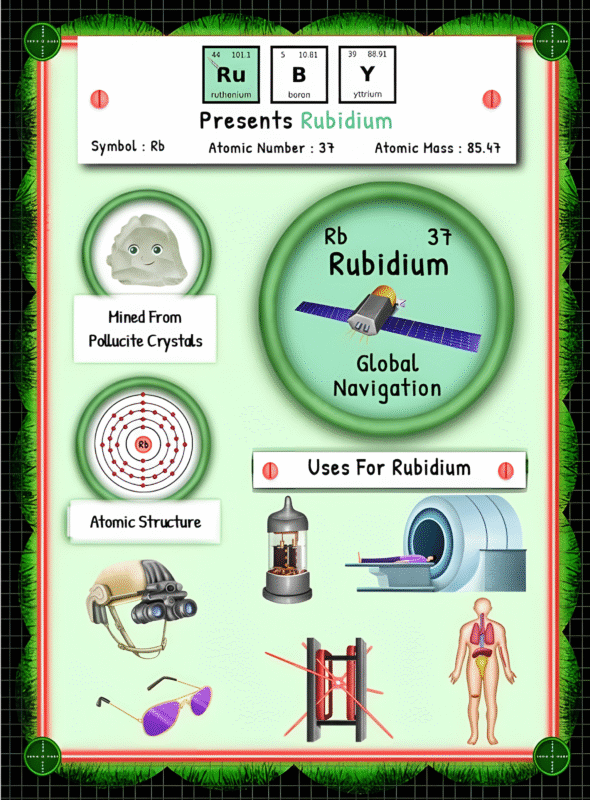
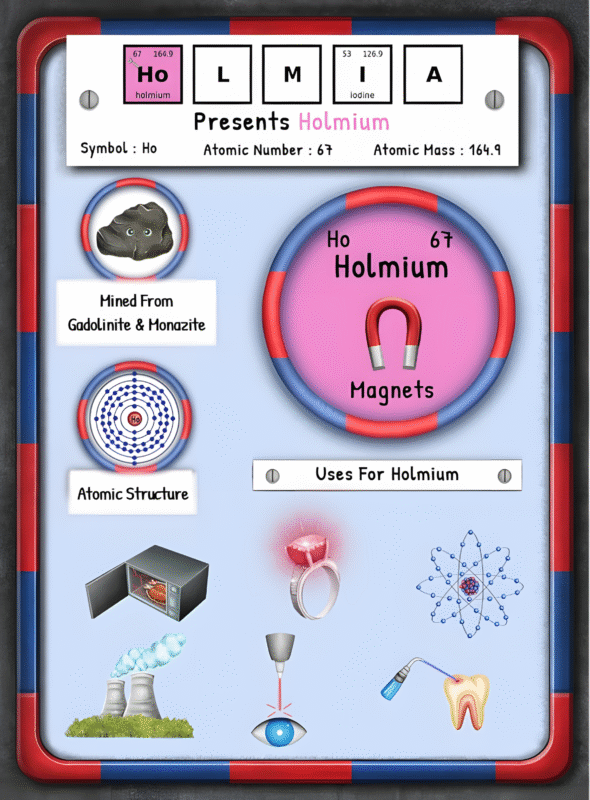
The Origin of Sodium: Occurrence, Extraction, Processing, and Environmental Impact

Sodium is an essential element in our daily lives, with various applications in industries ranging from food to medicine. Understanding the origin of sodium, its occurrence in nature, extraction methods, commercial processing, and environmental impact is crucial. This article aims to shed light on these aspects of sodium.
Occurrence:
Sodium, with the chemical symbol Na and atomic number 11, is one of the most abundant elements in the Earth’s crust. However, it does not occur naturally in pure elemental form due to its high reactivity. Rather, sodium occurs widely as compounds, most commonly as sodium chloride (NaCl), or table salt. This compound is found in large deposits around the world, such as rock salt formations and evaporite deposits.
Extraction and Mining Process:
As sodium does not naturally exist in pure form, extracting it from compounds like sodium chloride becomes essential. The mining process involves extracting NaCl-rich rocks from underground deposits or above-ground evaporite deposits. The most common mining method for sodium extraction is solution mining. This process involves injecting hot water into the salt deposit, dissolving the salt, and then pumping the saltwater to the surface. The resulting brine is further processed to obtain pure sodium chloride.
Commercial Processing:
The commercial processing of sodium involves several steps to obtain different forms suitable for various applications. Let’s take a closer look at the key processes:
- Sodium Compounds: Sodium is further processed into various compounds, such as sodium hydroxide (NaOH) and sodium carbonate (Na2CO3). These compounds have extensive applications in industries like chemicals, soaps and detergents, pulp and paper, and water treatment.
- Purification: The mined sodium chloride undergoes purification to remove impurities and minerals. This is achieved through crystallization, where the brine is cooled, causing the crystals to separate from the impurities. The pure crystals are then washed, dried, and sorted.
- Electrolysis: Electrolysis is a crucial step in the commercial production of sodium. The purified sodium chloride crystals are dissolved in water and subjected to electrolysis. This process separates sodium metal from chlorine gas by passing an electric current through the solution. Sodium ions are attracted to the negative electrode (cathode), where they give up an electron, forming metallic sodium. The chlorine ions travel to the positive electrode (anode) and evolve as chlorine gas.
The Leading Sodium Mining and Production Countries:
The distribution of sodium mining and production varies globally. The United States, China, and India are among the leading sodium mining countries, each contributing a significant share to the global production. The environmental impact of sodium mining varies depending on the geographical location and extraction methods used.
For instance, in the United States, the mining of sodium chloride is primarily carried out through solution mining and underground mining. This extraction process can have adverse environmental consequences such as land subsidence and groundwater contamination. On the other hand, China relies more on extracting sodium chloride from large, shallow evaporite deposits, which is considered a more environmentally friendly method.
China is also a major commercial producer of sodium compounds, mainly sodium carbonate. The production of sodium carbonate in China has both positive and negative environmental effects. On the positive side, the production process involves using limestone and salt, two abundant resources in China. However, the high energy consumption in sodium carbonate production contributes to China’s overall carbon emissions.
Conclusion:
Sodium, although abundant in nature, does not occur in pure elemental form. It is typically found as sodium chloride, or table salt, in large deposits worldwide. The extraction process involves mining sodium-rich rocks and using solution mining techniques to extract sodium chloride. Commercial processing includes purification of sodium chloride and subsequent electrolysis to obtain pure sodium. China, the United States, and India are major contributors to sodium mining and production, each having varying environmental impacts. It is crucial for countries to balance the demand for sodium with sustainable mining practices to minimize their environmental footprint in sodium production.
This article is brought to you by Sybrina Durant, the author of the middle grade picture book, Magical Elements of the Periodic Table Presented Alphabetically By The Elemental Dragons. Learn More. In that book Sodium is presented by the dragon, Sorn.
Inter-Active Elemental Fantasy-Themed Periodic Table from Magical Elements of the Periodic Table Presented Alphabetically by The Elemental Dragon Clan
Click here to use This Inter-Active Viewer To Learn More About The Elements Each Elemental Represents On This Periodic Table. Want this in a 24″ x 36″ Poster? Click here.
Sybrina Publishing Offers Fun Activities Based On The Book
Magical Elements of the Periodic Table Magical Elementals
Browse Magical Elemental Activities at MagicalPTElements or Sybrina-Publishing on TPT or Classful