The Origin of Nitrogen: A Key Element in Nature

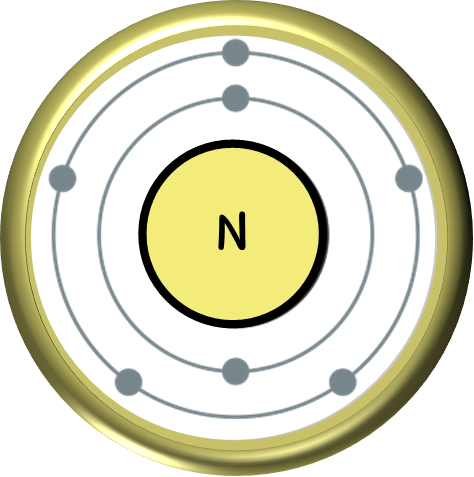
Nitrogen, symbolized by the letter N on the periodic table, is an essential element for life on Earth. It is the fourth most abundant element in the universe and plays a crucial role in both organic and inorganic processes. But where does nitrogen come from, and how is it obtained for commercial use? In this article, we will explore the origin of nitrogen, its presence in nature, the extraction and mining processes, commercial processing, and the countries that dominate its production and their impact on the environment.
Nitrogen does not occur naturally as a pure element. It is instead found in various compounds, such as ammonia (NH3), nitrate (NO3-), and nitrite (NO2-). These compounds can be derived from different sources, including the atmosphere, water bodies, and the Earth’s crust. The majority of nitrogen is present in the atmosphere, accounting for approximately 78% of its composition. However, atmospheric nitrogen is generally inert and unavailable to most organisms, leading to the necessity for its conversion into more usable forms.
One significant natural process that converts atmospheric nitrogen into a usable form is nitrogen fixation, which occurs through lightning strikes and certain types of bacteria. These bacteria, known as nitrogen-fixing bacteria or diazotrophs, have the ability to convert gaseous nitrogen into ammonia. This ammonia can then be utilized by plants and other organisms to synthesize organic compounds.
However, for commercial purposes, nitrogen is primarily obtained through mining and processing. One of the most common forms of nitrogen extraction is from the Earth’s crust, where it exists in the form of various minerals. Nitrate minerals, such as saltpetre (sodium nitrate) and Chilean nitrate (sodium nitrate and potassium nitrate), have historically been important sources of nitrogen for fertilizers and explosives.
The mining process for these minerals typically involves the extraction from underground or open-pit mines. Once the raw ore is obtained, it undergoes several stages of processing to remove impurities and isolate the desired nitrogen-containing compounds. This process can involve crushing, grinding, and further chemical treatments to produce a pure form of nitrogen that is suitable for commercial use.
Commercial processing of nitrogen further involves the conversion of nitrogen compounds into various products like ammonia, urea, and nitric acid. Ammonia is a vital precursor for the production of fertilizers, plastics, and other chemicals. Urea, on the other hand, is widely utilized as a fertilizer due to its high nitrogen content. Nitric acid finds applications in the production of explosives, dyes, and pharmaceuticals.
When it comes to the country that mines the most nitrogen, Chile takes the lead. With its abundant natural nitrate deposits, it has historically been a significant producer of nitrogen-based products. However, advancements in technology have shifted the production dominance, and today, China is one of the largest producers of nitrogen-based products globally, including ammonia and urea.
The extensive mining and production of nitrogen-based products can have significant environmental implications. In the case of Chile, excessive nitrate mining in the past led to environmental degradation and the depletion of natural resources. The overuse of nitrogen fertilizers can also result in water pollution through leaching and runoff, which can harm aquatic ecosystems and contribute to the formation of oxygen-deprived dead zones.
In conclusion, while nitrogen does not occur as a pure element in nature, it is abundant in various compounds. Nitrogen extraction involves mining from the Earth’s crust, particularly from nitrate minerals. Commercial processing plays a crucial role in converting nitrogen compounds into products such as ammonia, urea, and nitric acid. Countries like Chile and China have been significant players in nitrogen production, with notable environmental impacts resulting from intensive mining and excessive fertilizer use. As the demand for nitrogen-based products continues to grow, it becomes increasingly important to find sustainable methods of extraction and minimize the environmental footprint associated with its production.
This article is brought to you by Sybrina Durant, the author of the middle grade picture book, Magical Elements of the Periodic Table Presented Alphabetically By The Elemental Dragons. Learn More. In that book Nitrogen is presented by the dragon, Nitra.
Inter-Active Elemental Fantasy-Themed Periodic Table from Magical Elements of the Periodic Table Presented Alphabetically by The Elemental Dragon Clan
Click here to use This Inter-Active Viewer To Learn More About The Elements Each Elemental Represents On This Periodic Table. Want this in a 24″ x 36″ Poster? Click here.
Sybrina Publishing Offers Fun Activities Based On The Book
Magical Elements of the Periodic Table Magical Elementals
Browse Magical Elemental Activities at MagicalPTElements or Sybrina-Publishing on TPT or Classful